BALTIMORE, MD—Maryland Attorney General Anthony G. Brown has announced that the Consumer Protection Division’s Opioids Enforcement Unit has finalized settlements with Teva, Allergan, Walmart, and Walgreens, after multi-year investigations of these opioids-makers’ or chain pharmacies’ involvement in fueling the opioid crisis in Maryland.
The settlements are expected to provide $238 million to be used in Maryland’s and its subdivisions’ efforts to fight the opioid crisis. The settlements also force these companies to stop engaging in practices that gave rise to the opioid crisis and take steps to prevent further illegal conduct.
“The opioid epidemic has claimed thousands of innocent lives through addiction and overdoses, has torn families apart, and has devastated communities across this country,” said Attorney General Brown. “This settlement money will help support recovery efforts in Maryland and prevent future loss where we need it most. It also serves as a reminder that my Office will continue holding accountable those responsible for fueling the opioid crisis.”
According to court documents filed this morning in the Circuit Court for Frederick County, Teva marketed and sold extremely dangerous and addictive rapid-onset fentanyl products, including Actiq, a fentanyl lozenge that resembles a lollipop, and Fentora, a fentanyl tablet. Actiq and Fentora were appropriate and approved by the U.S. Food and Drug Administration only for the extreme cancer pain known as “breakthrough cancer pain” that arises from time to time in patients with advanced cancers that are unlikely to be cured. But Actiq and Fentora are not appropriate or approved for patients who do not have cancer, because they pose too much risk of addiction and death. Seeking to drive up its profits, Teva falsely claimed that the drugs were safe and appropriate for non-cancer conditions and funneled hundreds of thousands of dollars to at least 16 Maryland prescribers through a bogus speaker bureau, according to the complaint. The complaint alleges that these kickbacks encouraged these prescribers to prescribe Actiq and Fentora to consumers, most of whom did not have cancer and should not have taken Actiq or Fentora. A former sales representative who provided sworn testimony to the Attorney General’s Opioids Unit stated that Teva’s sales employees understood that these prescribers would prescribe fentanyl inappropriately if paid to do so, indicating that the prescribers he called on were “hypocrites” and “cowboys” and that the practice he called on that generated the most business for Teva had been known as “the graveyard of pain medicine.”
Another representative testified that Teva paid thousands of dollars in speaker fees, meals, and drinks to three prescribers from the same Annapolis practice who, according to Teva’s records, on at least 60 occasions took turns giving “educational” presentations about Actiq and Fentora, mostly to each other, their employees, and friends (including some doctors) who sometimes came along as attendees. These prescribers generally did not treat cancer patients; they treated chronic non-cancer pain. According to the complaint, at Teva’s direction, sales representatives also helped provide deceptive information to prescription plans to secure inappropriate coverage of the problematic prescriptions.
The complaint against Allergan charges that Allergan misled prescribers and patients about the relative safety of its extended-release morphine product, Kadian. Allergan sold Kadian by deceptively representing that Kadian was safer than other opioids. It also misled prescribers about the nature of addiction, claiming that addicted patients who were exhibiting signs of addiction were not really addicted but simply required more medicine to relieve their pain. These false messages led Maryland prescribers to increase opioids doses to those already suffering from addiction, contributing to the vast overprescription of opioids that fueled the opioids epidemic of addiction and death.
The Division’s complaints against Walmart and Walgreens charge that the pharmacy chains failed to live up to legal duties and their own representations that they would promote customers’ health and safeguard those customers from inappropriate or unsafe prescription drugs. Pharmacies are required by state and federal law to investigate opioid prescriptions that seem problematic before filling them, and Walmart and Walgreens promised their customers that they would do so. The complaints allege that contrary to their messages, however, both Walmart and Walgreens put inappropriate pressures on pharmacists and other pharmacy employees to fill prescriptions despite “red flags” that showed the prescriptions might be unsafe. According to the complaints, this led to Walmart’s 62 and Walgreens’ 186 stores in Maryland filling opioid prescriptions that were inappropriate and unsafe, creating or contributing to the addiction and ultimate death of many Marylanders. The complaints highlight, as an example, that Walmart and Walgreens pharmacies filled opioid prescriptions from healthcare providers at a pain management clinic in Baltimore County, which is no longer in business, for 39 and 116 patients of that practice, respectively, who later died from overdoses caused by opioids abuse and addiction. Walmart and Walgreens, the complaint charges, were aware of issues with the providers, but filled these and other prescriptions anyway, while deceiving the public that they were keeping consumers safe. The complaint against Walmart also charges that a Walmart in Western Maryland dispensed opioids prescriptions written by seven providers to 29 patients who ultimately died from overdoses involving opioids.
Maryland’s settlements are part of a broader collaborative effort with other states in which it and many Maryland subdivisions took part.
The projected revenue from each of the settlements is as follows:
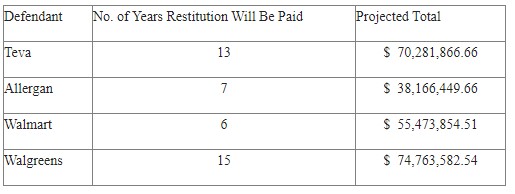
All revenue received by the State will be placed in the Maryland Opioid Restitution Fund, where it will be spent on efforts to ease the opioid crisis.
Photo by Alex Green from Pexels
Do you value local journalism? Support NottinghamMD.com today.

